The value of Dymista®
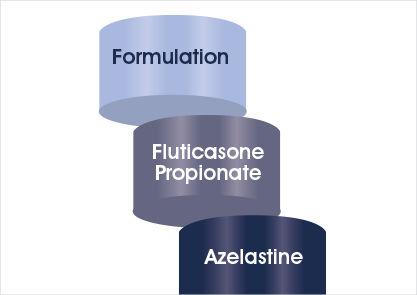
Multiple components in Dymista®
Multiple components contribute to the overall product effectiveness of Dymista®: the two active ingredients and the formulation.1

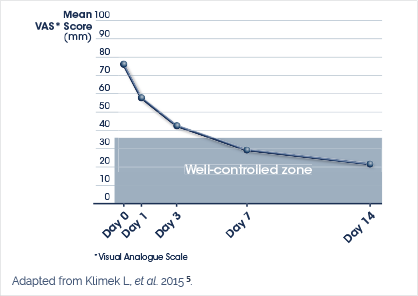
‘Dymista®’s dual therapy has been found to control symptoms more effectively and faster in patients with moderate and severe allergic rhinitis than intranasal azelastine or fluticasone propionate alone.’ 1,2
Dymista® has been shown to be twice as effective as fluticasone propionate or azelastine alone at treating the entire rhinitis symptom spectrum.3,4
For patients to get the full therapeutic benefit, regular usage of Dymista® nasal spray is essential and Dymista® is suitable for long-term use.7
Treatment should be continued for as long as the patient is exposed to the allergen(s).7
Adverse events: Include epistaxis, headache, dysgeusia and unpleasant smell. Hypersensitivity reactions include anaphylactic reactions, angioedema, bronchospasm, glaucoma, increased intraocular pressure, cataract, blurred vision, septal perforation, nasal irritation, nasal ulcers, throat irritation, nausea, dizziness, sleepiness, fatigue, rash, dry mouth and growth retardation may be possible in adolescents receiving prolonged treatment and growth should be monitored regularly.7
Warnings and precautions: Avoid concomitant use with ritonavir. Systemic effects of nasal corticosteroids may occur. Systemic exposure in severe liver disease may be increased. Monitor patients who experience changes in vision or have a history of ocular pressure, glaucoma and/or cataract. If adrenal function is impaired, take care when changing medication to Dymista®. In patients with infections, recent surgery or injury to nose or mouth, weigh benefits against the risks of use.7
Consult the Summary of Product Characteristics (SmPC) for more information.
Please refer to Summary of Product Characteristics (SmPC) before prescribing, particularly in relation to adverse reactions, precautions, contra-indications and method of use.
References
- Derendorf H, et al. Bioavailability and disposition of azelastine and fluticasone propionate when delivered by MP29-02, a novel aqueous nasal spray. Br J Clin Pharmacol. 2012; 74(1): 125-133.
- Meltzer E, et al. Clinically relevant effect of a new intranasal therapy (MP29-02) in allergic rhinitis assessed by responder analysis. Int Arch Allergy Immunol. 2013; 161(4): 369-77.
- Carr W, Bernstein J, Lieberman P, et al. A novel intranasal therapy of azelastine with fluticasone for the treatment of allergic rhinitis. J Allergy Clin Immunol. 2012; 129: 1282-1289.
- Meltzer EO, LaForce C, Ratner P, Price D, Ginsberg D, Carr W. MP29-02 (a novel intranasal formulation of azelastine hydrochloride and fluticasone propionate) in the treatment of seasonal allergic rhinitis: a randomized, double-blind, placebo-controlled trial of efficacy and safety. Allergy Asthma Proc. 2012; 33: 324-332.
- Klimek L, et al. Effectiveness of MP29-02 for the treatment of allergic rhinitis in real-life: results from a noninterventional study. Allergy Asthma Proc. 2015; 36(1): 40-47.
- Bousquet J, et al. Onset of Action of the Fixed Combination Intranasal Azelastine-Fluticasone Propionate in an Allergen Exposure Chamber. J Allergy Clin Immunol Pract. 2018; 6(5): 1726-1732.
- Dymista® Summary of Product Characteristics. Available at: https://www.medicines.org.uk/emc/product/9450. Last accessed: November 2023.
- Scadding GK, et al. BSACI guideline for the diagnosis and management of allergic and non-allergic rhinitis (Revised Edition 2017; First edition 2007). Clin Exp Allergy 2017; 47(7): 856‑889.
- Next-generation Allergic Rhinitis and Its Impact on Asthma (ARIA) guidelines for allergic rhinitis based on Grading of Recommendations Assessment, Development and Evaluation (GRADE) and real-world evidence. Available at https://www.jacionline.org/action/showPdf?pii=S0091-6749%2819%2931187-X. Last Accessed: November 2023.
- Scottish Medicine Consortium (SMC). Available at https://www.scottishmedicines.org.uk/medicines-advice/azelastine-hydrochloride-plus-fluticasone-propionate-dymista-abbreviatedsubmission-92113/ Last accessed: November 2023.
- Lipworth B, et al. An algorithm recommendation for the pharmacological management of allergic rhinitis in the UK: a consensus statement from an expert panel. NPJ Prim Care Respir Med. 2017; 27(1).
DYM-2023-0851 November 2023
Viatris Connect is an online platform for UK health Professionals.
Across the website you will find news, blogs and product information.
Register to Viatris Connect today
Please note that the website contains promotional and non-promotional material including educational content and resources to help you and your patients.
REGISTER NOW